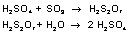Experiment C5.1.1.1 demonstrates the technical production of sulfuric acid by the contact method. The sulfur dioxide produced from the combustion of sulfur is oxidised by a catalyst to sulfur trioxide in the reaction tube. That is then led to water or sulfuric acid.
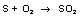
SO2 is oxidised out to SO3 on a catalyst.
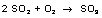
SO3 is easily soluble in concentrated sulfuric acid. This results in disulfuric acid, which can be transformed into sulfuric acid by the addition of water: